What is Plasma Medicine ?
Plasma medicine leverages a safe, ionized gas like helium or air to accelerate healing, control infections, and treat skin conditions without drugs or invasive procedures. By harnessing reactive oxygen and nitrogen species (RONS), plasma therapies stimulate the body’s natural repair processes and directly disrupt harmful pathogens.
How Does It Work?
Cold plasma interacts with tissue surfaces by generating reactive energy at the treatment site. This selectively affects pathogenic cells while preserving healthy tissue integrity. The result is a non-invasive, contactless therapy that can:
• Reduce microbial load
• Promote wound repair
• Stimulate cellular recovery mechanisms
These effects arise from controlled oxidative interactions rather than thermal damage, making plasma a distinct modality from lasers or electrocautery.
Why Does it Matter?
Traditional antibiotics and wound care methods are increasingly limited by resistance, slow healing times, and invasive procedures. Cold plasma offers a complementary and drug-free approach that can:
• Help manage antibiotic-resistant infections
• Accelerate healing of chronic wounds
• Treat dermatological conditions safely and effectively
This technology opens new pathways in clinical care while reducing dependence on systemic drugs.
Why Plasma
A Different Way to Interact with Biology, Modern medicine relies on molecules.
Drugs require receptors. Chemistry requires pathways, Plasma operates differently.
It introduces physical energy at the biological interface using electric fields, reactive species, and charge interactions. This creates new ways to influence cells, microbes, tissues, and environments.
Plasma does not replace biology. It expands what biology can do.
At JivaJet, we're pioneering the use of cold plasma technology to revolutionize medical treatments.
Our focus is on developing innovative solutions that address antibiotic-resistant infections and promote rapid wound healing, offering drug-free, painless, and cost-effective therapies.
Mechanism
- Infected tissues exhibit higher oxidative stress and unique RONS (Reactive Oxygen and Nitrogen Species) profiles compared to healthy cells.
- JivaJet applies cold plasma—containing RONS and electromagnetic (EM) waves—for 5–10 minutes at the site.
- This exposure pushes diseased cells beyond their oxidative threshold, triggering apoptosis.
- Healthy cells withstand the brief oxidative spike, self-repair, and return to homeostasis.
- Plasma-induced cell death mechanisms include: DNA damage only for Pathogens
- Membrane blebbing in microbial cells – After eliminating the microbial infection, cell proliferation and healing occur at approximately twice the normal rate compared to without exposure to JivaJet’s plasma.
- Cold Atmospheric Plasma (CAP)
- Generates Oxygen and Nitrogen species
- Treats broad range of bacterial skin infections
- Promotes rapid wound healing
- FDA tested
Advantage
- Topical, non-Invasive, Painless
- Low cost
- Patented
- Rapid recovery time
- Reduce reliance on antibiotics
JivaMed is currently in the pre-revenue and pre-clinical research and development stages. Any images, animations, or computer-generated renderings shown are intended to illustrate envisioned features, potential capabilities, and future use cases of JivaJet’s plasma-based technologies. These products have not been reviewed or approved by the U.S. FDA and are not currently available for commercial sale. All forward-looking statements are based on preliminary research and management’s current understanding, and are subject to change without notice.
Fields of Use
Cancer / Oncology
Translational Plasma Research in Oncology
Cancer is characterized by abnormal cellular metabolism, dysregulated growth signaling, and altered oxidative stress tolerance. These features make malignant tissue particularly sensitive to localized, controlled biological stressors.
JivaJet is advancing cold atmospheric plasma as a translational research modality to study targeted biological interactions in oncology across in-vitro systems, animal models, and early clinical investigation.
Research Progress
Plasma oncology research includes:
• In-vitro studies across multiple cancer cell lines
• Pre-clinical mouse models, including glioblastoma
• Investigation of plasma-induced apoptosis and stress signaling
• Surface and microenvironment-level tumor interaction models
This work builds on plasma-based tumor ablation research originating from the same laboratory ecosystem.
Clinical Research Context
Certain oncology-related plasma investigations have progressed to early human feasibility studies under institutional approval. These efforts focus on safety, biological interaction, and mechanistic understanding rather than therapeutic outcomes.
Cold plasma is not positioned as a standalone cancer treatment and remains under active clinical investigation.
Rare Genetic Disorders (NF1 & TSC)
Early Clinical Research for Genetic Tumor Syndromes
Neurofibromatosis Type 1 (NF1) and Tuberous Sclerosis Complex (TSC) are genetic disorders associated with abnormal cell growth, tumor formation, and dysregulated signaling pathways. Management options are often limited, particularly for localized or surface-accessible lesions.
JivaJet is investigating cold atmospheric plasma as a localized bio-interaction modality for NF1 and TSC through translational and early clinical research.
Research Progress
Work in NF1 and TSC includes:
• In-vitro cellular studies
• Pre-clinical animal models
• Institutionally approved first-in-human feasibility studies
• Evaluation of plasma-tissue interaction, safety, and response
These studies aim to understand how genetically altered tissue responds to controlled plasma exposure under clinical research conditions.
Clinical Context
Plasma technologies described are being evaluated for feasibility and safety in human research settings. They are not FDA-approved treatments and are not marketed as therapeutic interventions.
Any future clinical application would require additional trials and regulatory clearance.
Cold plasma is being actively studied for its ability to selectively induce stress responses in malignant cells while minimizing damage to surrounding healthy tissue. Plasma-generated reactive species interact with cancer cells’ altered metabolic and redox states, making them more susceptible to oxidative disruption.
Rather than acting as a drug or radiation source, plasma functions as a localized bio-interaction modality, enabling controlled exposure at the tissue surface.
Areas of investigation include
• Adjunctive oncology research
• Localized tumor surface treatment models
• Post-surgical margin management research
• Plasma-induced apoptosis pathways
• Combination approaches with existing therapies
Anti-Microbial Resistance / Multi-Drug Resistance
Plasma-Based Antimicrobial Research Beyond Antibiotics
Antimicrobial resistance and multidrug-resistant infections continue to outpace the development of new antibiotics, creating significant challenges across healthcare, veterinary medicine, and environmental control. Resistant pathogens often persist through biofilm formation and reduced susceptibility to conventional chemical agents.
JivaJet is advancing cold atmospheric plasma as a non-antibiotic antimicrobial research modality that enables localized biological inactivation through physical and chemical interactions rather than pharmacological mechanisms.
Research Progress
Antimicrobial plasma research includes:
• In-vitro validation against drug-resistant organisms
• Biofilm disruption studies at the surface level
• Plasma-induced microbial membrane and DNA damage analysis
• Exploration of resistance-agnostic antimicrobial mechanisms
Plasma-generated reactive oxygen and nitrogen species act through multi-target pathways, reducing the likelihood of resistance development.
Translational Context
Plasma-based antimicrobial technologies are being evaluated as complementary infection-control strategies rather than replacements for antibiotics. All AMR/MDR applications remain investigational and are not FDA-approved for clinical use.
40% of MRSA cases require surgery — antibiotics are increasingly ineffective. Combat pathogens with non-antibiotic treatment
Rising resistance in common pathogens (like MRSA, Acinetobacter baumannii) creates significant treatment challenges. Plasma medicine provides a localized, non-antibiotic means to control infection at the skin and tissue interface.
Treat drug-resistant infections in pets without antibiotics or surgery. 25% of dogs and 15% of cats in the U.S. suffer from recurring skin infections. Recurring skin infections in humans and animals can result from biofilm formation and resistance. Plasma-based therapies offer a non-painful, outpatient treatment option that supports tissue regeneration.

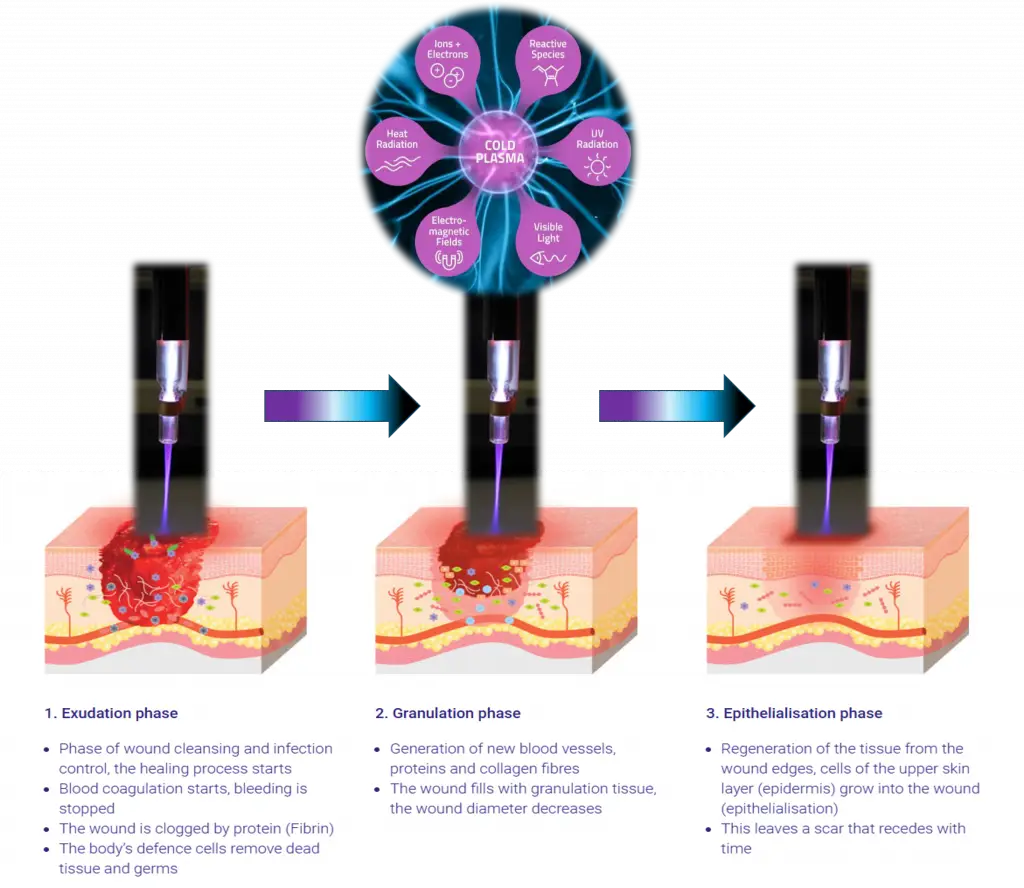
Wound Healing
Plasma-Assisted Tissue Response and Regeneration Research
Chronic and non-healing wounds are often driven by a combination of microbial burden, impaired cellular signaling, and reduced tissue regeneration capacity. These wounds frequently fail to respond to standard care, resulting in prolonged healing times and increased complication risk.
JivaJet investigates cold atmospheric plasma as a localized, non-invasive research platform to study how controlled plasma exposure influences wound environments and tissue response.
Research Progress
Plasma wound-healing research includes:
• Pre-clinical animal models evaluating wound closure dynamics
• Plasma effects on fibroblast migration and proliferation
• Reduction of microbial load at wound surfaces
• Investigation of oxygenation and signaling pathways involved in tissue repair
Plasma enables surface-level biological interaction without thermal damage or chemical exposure.
Clinical Context
Wound-healing applications of plasma are under translational investigation and are not approved clinical therapies. Research focuses on biological response, safety, and feasibility rather than clinical outcomes.
Chronic wounds like diabetic ulcers affect millions and often fail to heal with standard of care.
Conditions such as diabetic ulcers and pressure sores often fail to heal with standard care.
Cold plasma supports rapid re-epithelialization and reduce recovery times.
Decontamination & Sterilization
Plasma for Rapid, Chemical-Free Decontamination
Cold atmospheric plasma offers a non-chemical, non-thermal approach to surface and equipment decontamination. By generating reactive oxygen and nitrogen species at ambient temperatures, plasma disrupts microbial membranes, proteins, and genetic material without damaging underlying materials.
Unlike traditional sterilization methods that rely on heat, chemicals, or prolonged exposure times, plasma enables rapid, localized decontamination suitable for sensitive environments.
Potential use cases include
• Medical equipment and device surfaces
• Hospital rooms and high-touch areas
• Laboratory containment and biosafety workflows
• Emergency response and field decontamination
• Spaceflight and planetary protection protocols
Plasma decontamination is particularly valuable where chemical residues, moisture, or heat exposure are unacceptable.
Key distinction: Plasma does not “clean” surfaces — it inactivates biological threats at the molecular level.
Why Plasma Decontamination Matters
Modern healthcare and research environments face rising challenges from:
• Antibiotic-resistant organisms
• Persistent biofilms
• Chemical sterilant toxicity
• Time-intensive decontamination cycles
Plasma offers a complementary solution that can reduce reliance on chemical disinfectants while improving turnaround times and safety profiles.
Astrobiology & Seed Germination
Plasma Research for Life in Extreme Environments
Astrobiology explores how biological systems respond, adapt, and survive in extreme environments beyond Earth. These environments are characterized by radiation exposure, low pressure, limited nutrients, and chemically hostile substrates such as lunar and Martian regolith.
JivaJet is investigating cold atmospheric plasma as a research tool to study how controlled energy exposure influences seed germination, early plant development, microbial interactions, and overall biological resilience under simulated extraterrestrial conditions.
Why Plasma Matters in Astrobiology
In space and planetary environments, traditional biological and agricultural processes are constrained by:
• Sterile or nutrient-poor substrates
• Microbial imbalance or contamination risks
• Environmental stressors that inhibit growth
• Limited ability to intervene with chemicals or heat
Plasma offers a non-thermal, non-chemical approach to influence biological systems at the surface level, making it uniquely suited for astrobiology and space-biology research.
Research Focus Areas
Current areas of investigation include:
• Plasma-assisted seed surface activation
• Germination behavior in lunar and Martian soil simulants
• Microbial load management without chemical sterilants
• Early-stage plant stress-response signaling
• Biological resilience under simulated space conditions
• Biological interaction studies relevant to closed-loop life-support systems
This research seeks to understand how plasma-mediated surface interactions may support biological viability in extreme and resource-constrained environments.
Scientific Context
Plasma is not used to genetically modify seeds or organisms.
Research focuses on physical and biochemical surface interactions, including changes in wettability, microbial reduction, and early-stage biological signaling that may influence germination and early growth outcomes.
Why This Research Is Important
Astrobiology and space-based biological research are critical to:
• Long-duration human spaceflight
• Lunar and planetary habitation
• Closed-loop life support systems
• Space medicine and biosafety
Understanding how plasma interacts with biological systems in extreme environments may inform future strategies for sustainable life beyond Earth.
Development Status
All astrobiology and seed germination research described is pre-clinical and investigational. Plasma technologies referenced are not approved for agricultural or medical use and remain subject to ongoing research and validation.
